
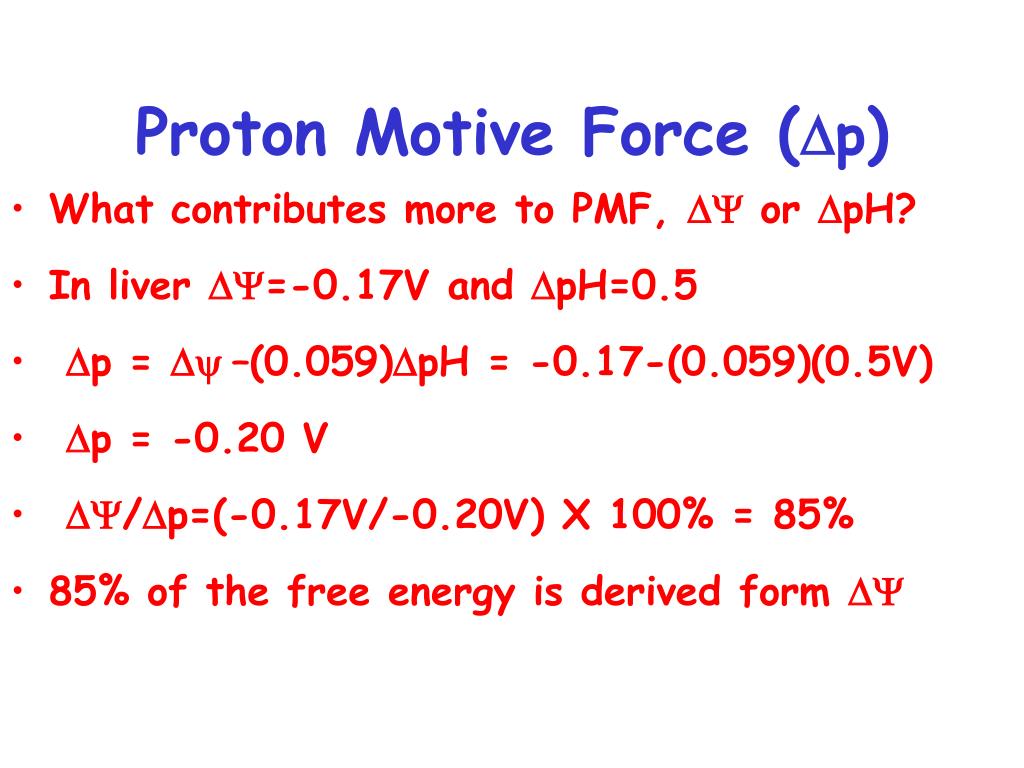
ATP cannot be stored and so its synthesis is closely linked to its consumption. This implies every ATP molecule must be reused 1000 or more multiple times in a day. The energy utilized every day by an adult requires the hydrolysis of 100 to 150 Moles of ATP.
The aggregate amount of ATP in the human body is about 0.1 Mole. Įstimates show that the normal body uses 40 kg of ATP on a daily basis hence ATP production is one of the most frequent processes occurring in the body. The ubiquitous distribution of ATP allows for signaling functions that have a uniquely broad influence on physiological functioning. Khakh and Burnstock, established that ATP also serves as a critical signaling molecule that allows inter and intracellular communications. Besides, ATP has crucial role in RNA and DNA synthesis and in amino acid activation during protein synthesis. Other than supporting almost all the cellular functions that require energy, ATP also works as a coenzyme during phosphorylation reactions. ADP can absorb energy and regain the group to regenerate an ATP molecule to maintain constant ATP concentration. Hydrolysis of the third phosphate group produces adenosine diphosphate (ADP) and inorganic phosphate (Pi), along with considerable release of energy. In an ATP molecule, two high-energy phosphate bonds, called phosphoanhydride bonds, are responsible for high energy content.
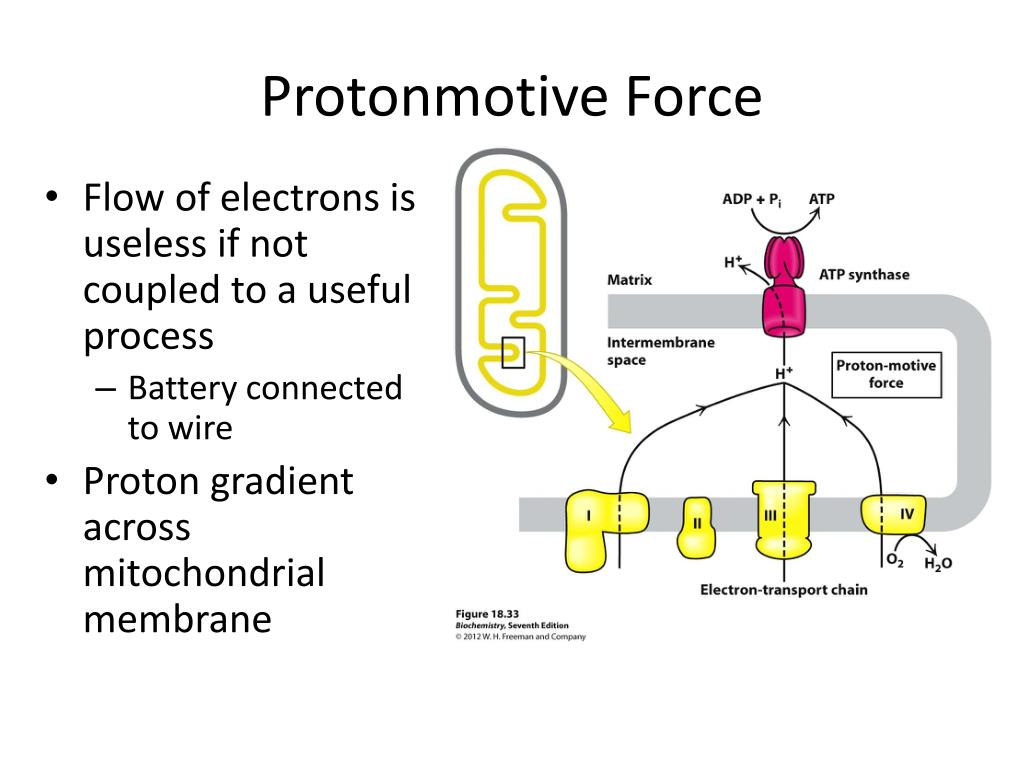
ATP is the fuel for the operation of almost all metabolic pathways of the cell. ATP generation is the principle energy generating procedure found in all forms of life.
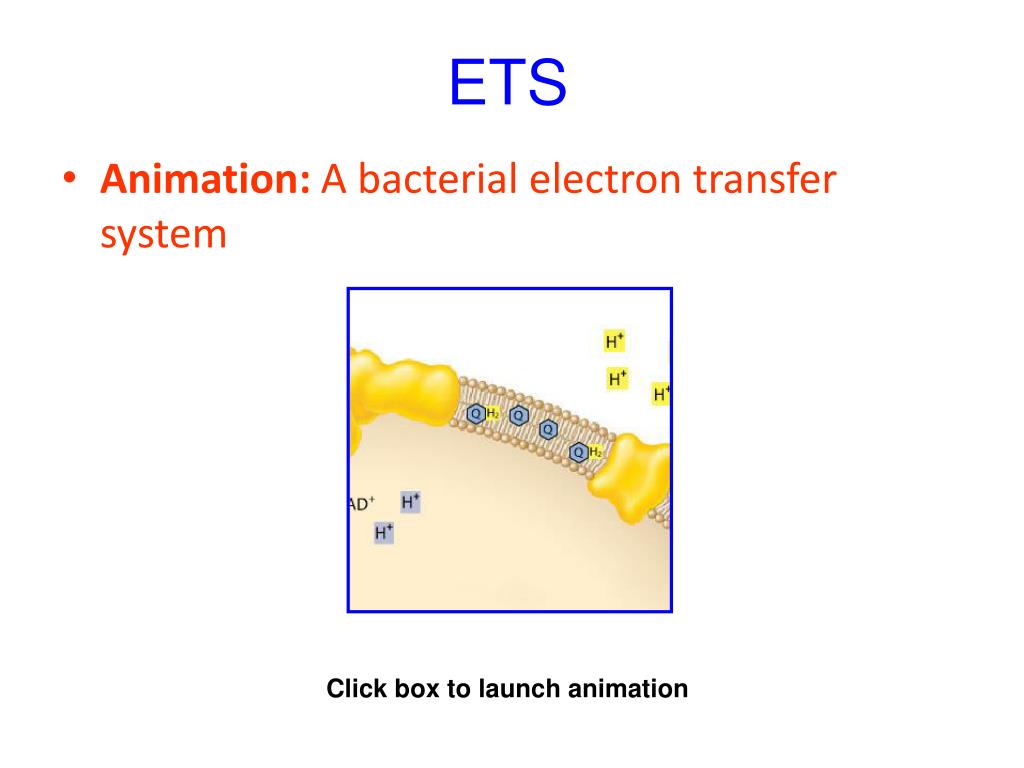
Often referred as “molecular currency” for intracellular energy transfer, Adenosine Triphosphate (ATP) functions as a chemical fuel by powering many organic processes of life.


 0 kommentar(er)
0 kommentar(er)
